University of Arkansas, NOWDiagnostics Partner for COVID-19 Antibody Test and Prevalence Study
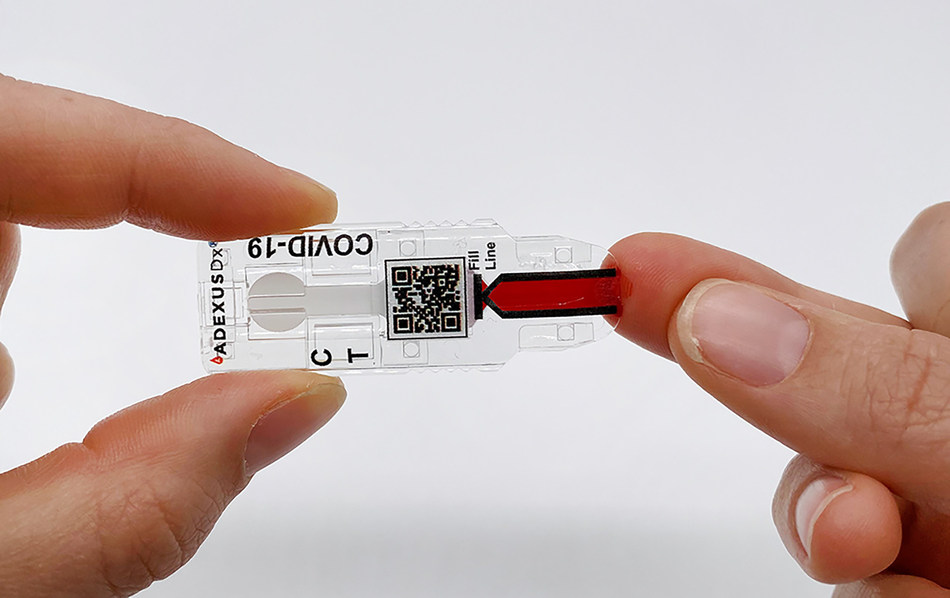
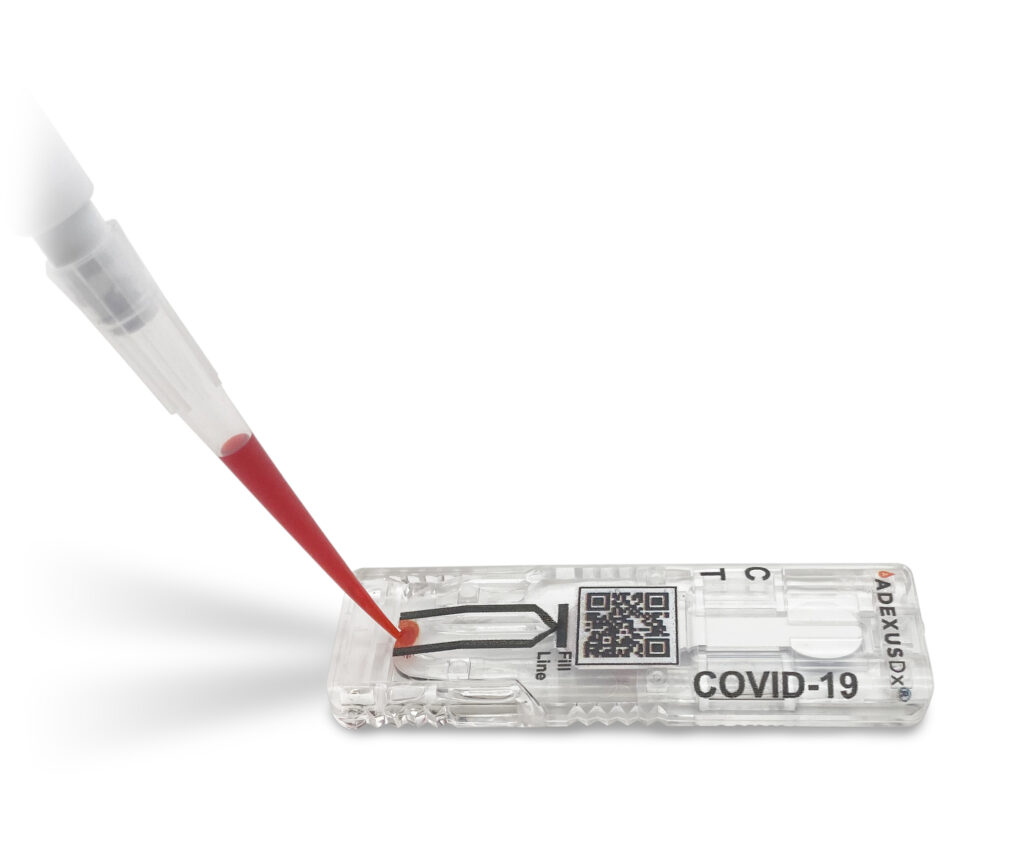
SPRINGDALE, Ark., June 16, 2021 /PRNewswire/ — The University of Arkansas and NOWDiagnostics, Inc., a Springdale-based leader in innovative diagnostics testing, announced today an active partnership to study the prevalence of SARS-CoV-2 virus antibodies among University of Arkansas (U of A) students, staff and faculty. The ADEXUSDx® COVID-19 Test is a rapid serology, self-contained assay […]