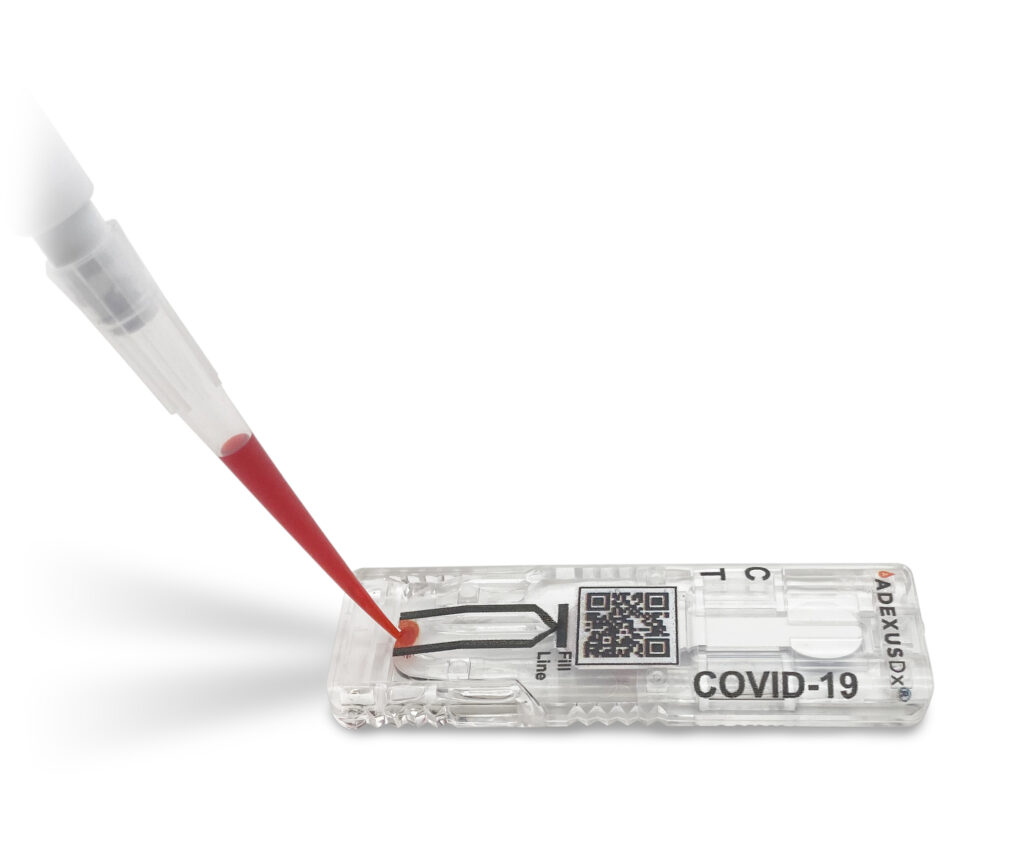
NOWDx seeks FDA Emergency Use Authorization (EUA) for COVID-19 Test
The ADEXUSDx® COVID-19 Test is a rapid serology test that detects total antibodies to SARS-CoV-2. It provides in-field, lab accurate results within 15 minutes, using as little as a drop of blood. The test does not require buffers or reagents, lab equipment, refrigeration, or in demand ancillary items. On May 29th, NOWDiagnostics filed for Emergency Use Authorization (EUA) and is currently pending review.
Interested in learning more? You can read the full article here.
NOWDiagnostics, Inc., based in Springdale, Arkansas, is a leader in innovative diagnostics testing. Its ADEXUSDx® product line features a lab at your fingertip, using a single drop of blood to test for a variety of common conditions, illnesses, and diseases, with results in a matter of minutes. By eliminating the need to send tests to off-site laboratories, NOWDiagnostics products have the potential to decrease the waiting period to determine test results by days. For more information about NOWDiagnostics, visit www.nowdx.com. For more information about the ADEXUSDx® COVID-19 Test, including its intended use, features, benefits and limitations, and directions for use, visit www.c19development.com. The ADEXUSDx® COVID-19 Test will be distributed by C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics. Laboratories may contact www.c19development.com/order to place an order.