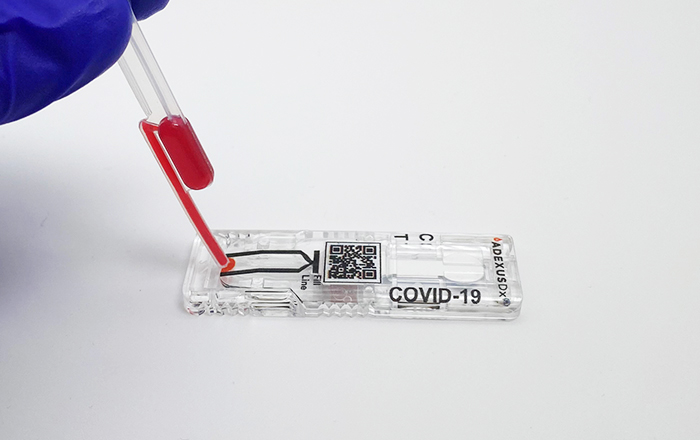
American Pharmacists Association Foundation, NOWDiagnostics Launch Nationwide In-Pharmacy Real-Time COVID-19 Antibody Testing with Spike Protein-Targeted, Rapid ADEXUSDx® Test
SPRINGDALE, ARK., July 19, 2021 – The American Pharmacists Association Foundation (APhA Foundation) and NOWDiagnostics, Inc., a Springdale-based leader in innovative diagnostics testing, today announced that NOWDiagnostics’ ADEXUSDx® COVID-19 antibody test, a rapid-results self-contained fingerstick test, that can be used at point of care, that accurately detects the presence of COVID-19 antibodies in 15 minutes, […]