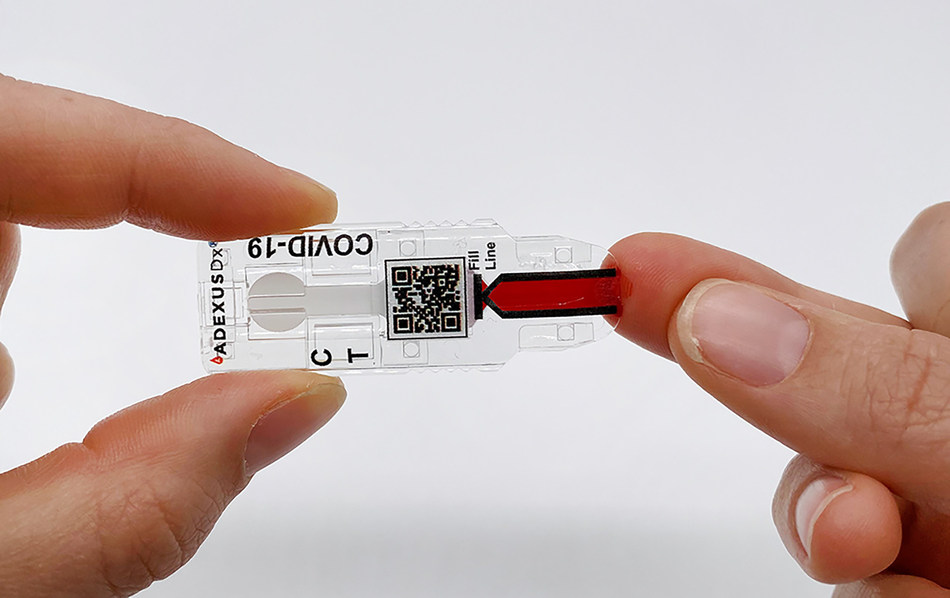
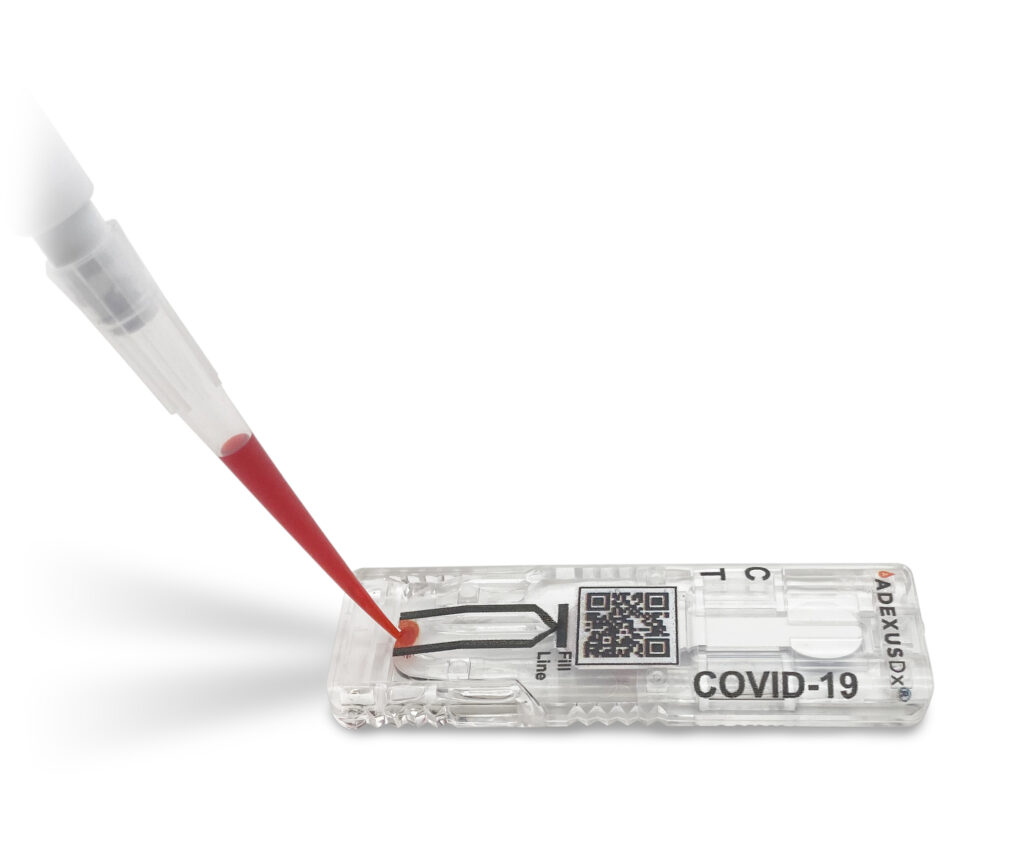
NWA’s NOWDiagnostics Chosen as Endeavor Entrepreneur at 7th Virtual International Selection Panel
Bentonville, AR — Endeavor concluded the 7th Virtual International Selection Panel (ISP) with the selection of nine new high-impact entrepreneurs representing 1,310 companies from 37 markets around the world, including Northwest Arkansas’ Now Diagnostics entrepreneurs, Kevin Clark and Jeremy Wilson. NOWDiagnostics is a developer and manufacturer of medical devices that deliver an innovative diagnostics testing […]