COVID-19 Antibody Test
FDA Emergency Use Authorized & CE Marked
The ADEXUSDx® COVID-19 Test is an in vitro lateral-flow immunoassay intended for qualitative detection of total antibodies to SARS-CoV-2 in human venous whole blood (dipotassium EDTA) and plasma (dipotassium EDTA), serum, and fingerstick whole blood.
The ADEXUSDx® COVID-19 Test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies conders protective immunity. The ADEXUSDx® COVID-19 Test should not be used to diagnose or exclude acute SARS-CoV-2 infection.
Available in:
- Box of 25
- Box of 50
- Box of 25 w/ Lancets
- Box of 50 w/ Lancets
- Case of 1,000
- Test Control
The ADEXUSDx® COVID-19 Test is an in vitro lateral-flow immunoassay intended for qualitative detection of total antibodies to SARS-CoV-2 in human venous whole blood (dipotassium EDTA) and plasma (dipotassium EDTA), serum, and fingerstick whole blood.
The ADEXUSDx® COVID-19 Test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies conders protective immunity. The ADEXUSDx® COVID-19 Test should not be used to diagnose or exclude acute SARS-CoV-2 infection.
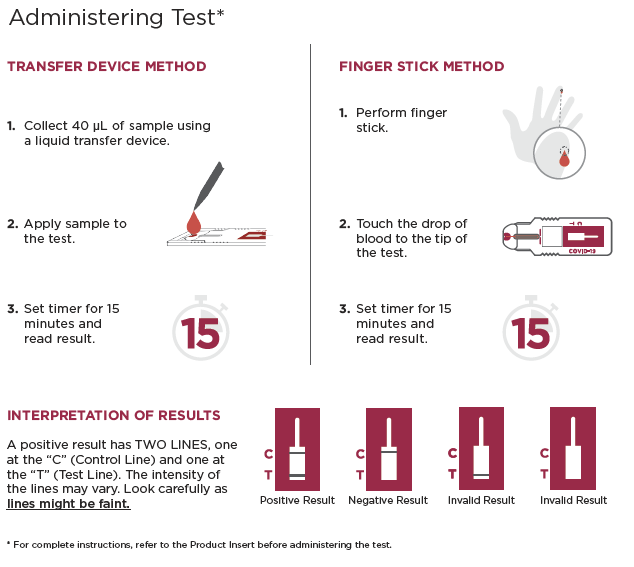
| Time to Result | 15 minutes |
| Result Window | Read before 30 minutes |
| Storage Conditions | Room temperature storage or 15°C – 30°C (59°F -86°F) |
| Operating Temperature | Room temperature storage or 15°C – 30°C (59°F -86°F) |
| Test Shelf Life | 18 months* |
| Sample Type(s) | Human whole blood or plasma (EDTA) and serum |
- No buffers or external reagents needed
- No lab equipment
- No in demand ancillary items
- No refrigeration
- Highly sensitive and specific
- Detects total antibodies (IgG, IgM, and IgA)
- Used to identify adaptive immune response (indicating recent or prior infection)
Where can I use the Test?
Test of venous whole blood (dipotassium EDTA), plasma (dipotassium EDTA), and serum specimens is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform moderate or high complexity tests.
Testing of fingerstick whole blood specimens is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform high, moderate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
What sample types are approved for use with the test?
Approved sample types include human venous whole blood (dipotassium EDTA) and plasma (dipotassium EDTA), serum, and fingerstick whole blood.
Is this Technology FDA Approved?
This product has not been FDA cleared or approved but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories.
This product has been authorized only for detecting the presence of total antibodies to SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the diagnostic for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Why should you be tested?
Antibody or Serology tests search for and detect antibodies found in your blood to assess if you have been previously infected with the virus that causes COVID-19.
- An antibody is a protein found in the blood that is produced as a response to counteract and defend the body against a specific type of antigen.
- Antibodies are produced by your immune system immediately after you have been infected with the virus or have received a vaccination.
- Antibodies activate distinct systems in the body to destroy foreign bacteria or viruses, which can help to fight off infections and protect you from getting that disease again.
- The length of protection from antibodies varies for each person and disease.
Antibody tests are not to be used to establish a current COVID-19 virus infection, except in the instance viral testing was deferred. Antibodies can take 1-3 weeks after the infection to be produced and may not display if you are currently infected.
How is our test different from other antibody tests?
There are many differentiators:
- Unlike other rapid tests on the market, our test was developed and is manufactured in the United States.
- Unlike many rapid tests on the market, our test is a total antibody test designed to detect all human immunoglobulins, including IgG, IgM, and IgA, which may indicate recent or prior exposure to SARS-CoV-2 (the virus that causes COVID-19).
- Due to our patented technology, our test is highly sensitive and specific.
- No transport media, buffers, or in-demand ancillary items are needed.
- Unlike other tests that require expensive lab equipment and specialized training personnel, our test can be administered in-field.
Why are you detecting total antibodies? Why not IGG, IGM separately?
Current research into the antibody response in COVID-19 patients has indicated that the classical antibody pattern of IgM first then IgG later is not followed. There has been evidence of IgG antibodies first, concurrent development of IgG and IgM antibodies, as well as IgM antibodies first. There is no established clinical utility for distinguishing between IgG and IgM antibodies. Our test is strategically designed to detect total immunoglobulin (IgG + IgM + IgA) in an effort to maximize test sensitivity for identifying people who have been infected with COVID-19.
Please click to view or print:
- ADEXUSDx COVID-19 Test Quick Reference Instructions
- EUA201531-S002 NOWDiagnostics IFU 09-22-2021
- ADEXUSDx COVID-19 Control Set Product Insert
- ADEXUSDx COVID-19 Healthcare Fact Sheet
- ADEXUSDx COVID-19 Recipient Fact Sheet
- EUA201531-S002 NOWDiagnostics Reissue Letter of Authorization 09-22-2021 FINAL
- ADEXUSDx COVID-19 Test Product Brochure