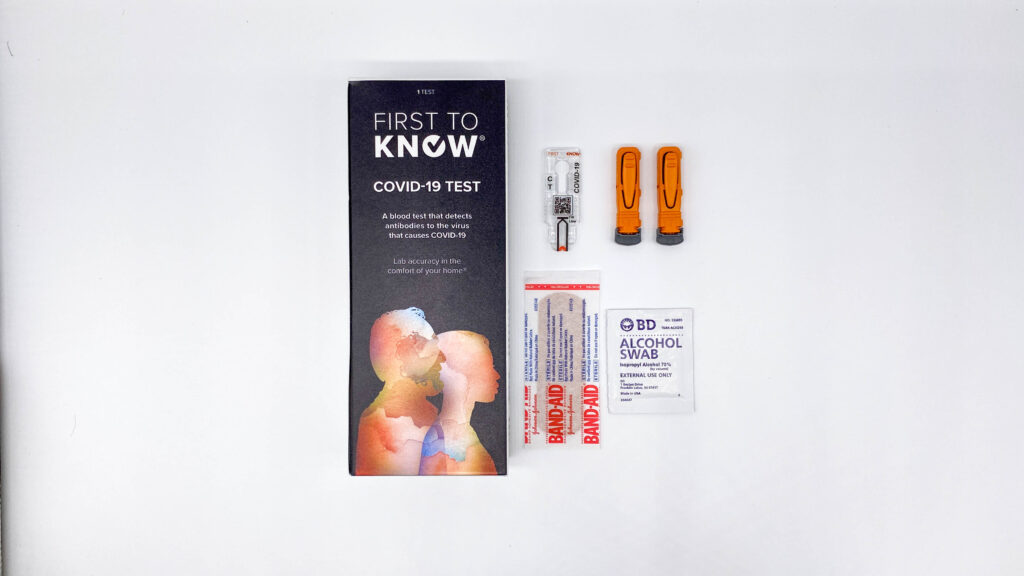
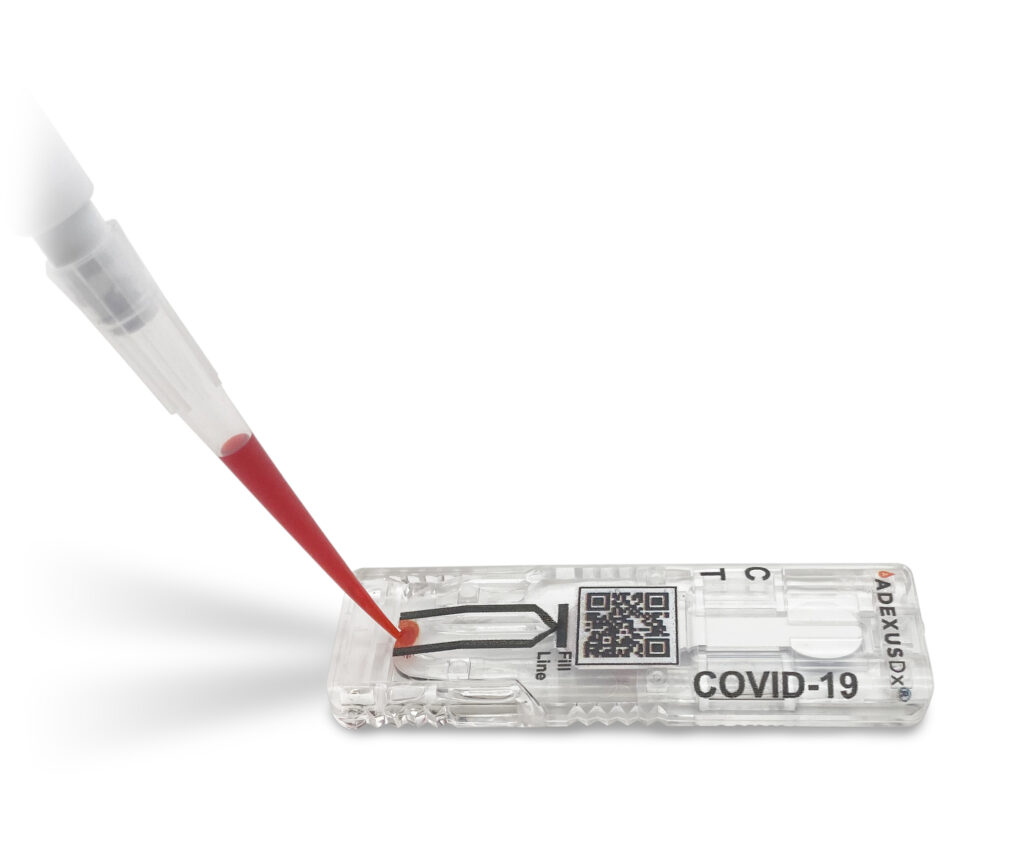
Springdale company creates rapid COVID-19 antibody test
Now Diagnostics says this test can be done at home by anyone. All you do is prick your finger and wait 10 minutes for the results. SPRINGDALE, Ark. — A Northwest Arkansas company has created a rapid COVID-19 antibody test that has approval by the European Union. “It’s pretty amazing, I’m really proud of the […]
Springdale company creates rapid COVID-19 antibody test Read More »